Tel. +49 (0) 4832 – 9786000
Email info@valeo-one.de
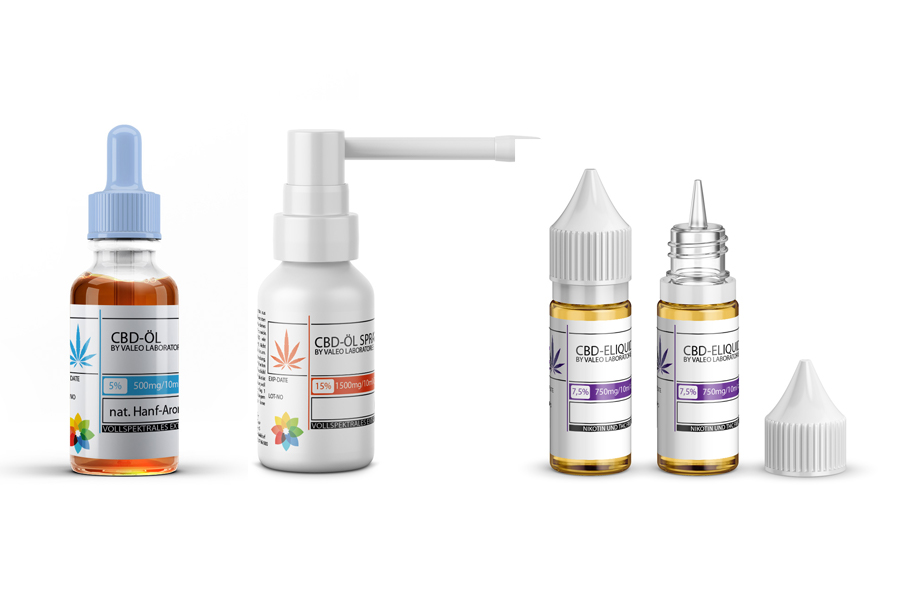
Valeo Laboratories - CBD, eLiquid & Nicotine-Salt production from Germany
Founded in 2010, Valeo Laboratories are engaged in the development and production of e-liquids for electronic cigarettes. A special feature is that apart from the company’s own development department, this was the first time that e-liquids were produced in Germany using only German ingredients. Valeo GmbH is certified by the Schleswig-Holstein Ministry of Energy Transition, Agriculture, Environment and Rural Areas for the production of nicotine-containing liquids for e-cigarettes as well as a registered producer for CBD containing products.
Where and what we produce:
Valeo Laboratories
Valeo GmbH
Gildeallee 5
D-25704 Meldorf / Germany
Tel: +49 4832 9786000
Valeo Laboratories product listing:
e-Liquids with nicotine
e-Liquids with nicotine-salt
e-Liquids with nicotine-substitute BioNic
e-Liquids with CBD | Cannabidiol
Shake´n Vape – 60ml & 120ml
Nicotine Booster
Nicotine-Salt Booster
Flavour concentrates
CBD-Oil fullspectrum, THC free
CBD-Oil crystalline isolate, THC free
CBD crystals, THC free
CBD capsules, THC free
CBD cremes, THC free
CBD balms, THC free
CBD lotions, THC free
E-mail: info@valeo-laboratories.com
Valeo GmbH
Gildeallee 5
D-25704 Meldorf / Germany
Tel: +49 4832 9786000
Valeo Laboratories product listing:
e-Liquids with nicotine
e-Liquids with nicotine-salt
e-Liquids with nicotine-substitute BioNic
e-Liquids with CBD | Cannabidiol
Shake´n Vape – 60ml & 120ml
Nicotine Booster
Nicotine-Salt Booster
Flavour concentrates
CBD-Oil fullspectrum, THC free
CBD-Oil crystalline isolate, THC free
CBD crystals, THC free
CBD capsules, THC free
CBD cremes, THC free
CBD balms, THC free
CBD lotions, THC free
E-mail: info@valeo-laboratories.com
Request our CBD & Vape product catalog

Areas of expertise
The Valeo Laboratories develop with their customers the best liquid for individual market needs.
Branding: the Valeo marketing team helps you to develop your corporate identity with your individual brand of liquid.
Benefit from our experience of selling in 35 countries around the world to choose the range that is best for you.
Win a decisive market advantage by using an innovative liquid manufacturer who is always researching new developments.




